
Innovation
-
Wound Exudate Management Technology
On Nov. 13, 2009, the FDA issued a Preliminary Public Health Notification*1 and Advice for Patients on serious complications, especially bleeding and infection, from the use of NPWT systems. FDA has received reports of six deaths and 77 injuries associated with NPWT systems from January 1, 2007 to November 13, 2009.
On February 24, 2011,FDA issued UPDATE on Serious Complications Associated with Negative Pressure Wound Therapy Systems*2, FDA received reports of an additional six deaths and 97 injuries, for a total of 12 deaths and 174 injury reports since 2007.
The statistics of NPWT adverse report before 2007 published on FDA website shows that 13 deaths and 61 injuries were reported in America. And during February 24, 2011 and July 1, 2012, another 5 death reports are published on FDA website.
15 of the 30 deaths were associated with bleeding.
With the innovative exudate management technology, Foryou NPWT system can monitor real-time exudate volume. Visual and auditory alarm will be triggered and the therapy will stop automatically for potential bleeding, which alerts doctors to take measures timely.
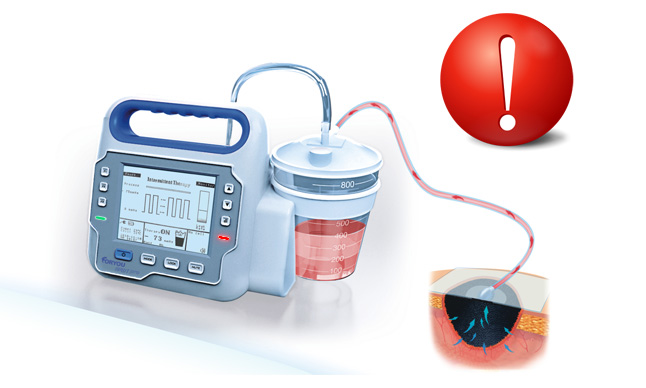
*1http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm190658.htm
*2http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htm
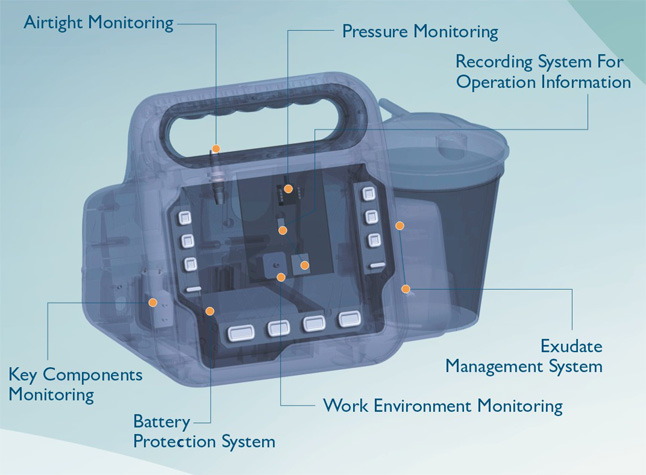
Key Components Monitoring & Protection
In March, 2009, the U.S. Department of Health and Human Services reported NPWT device malfunctions for 13 percent (28 of 215) of the beneficiaries. In most cases, the NPWT suppliers replaced the devices. Suppliers noted that a few beneficiaries had problems with multiple devices, necessitating a number of replacements.
Some so-called NPWT device is modified from cheap suction machine which is lack of accurate pressure monitoring. It would easily lead to excessive pressure and adverse events. The principle of suction machine is different from that of NPWT device. The parts and components of suction machines would become overheated after a period of operation which may easily damage the device and even cause explosion. Moreover, the noise of suction machine is much bigger than that of NPWT, which may affect patient’s quality of life.
The key components monitoring & protection function of Foryou NPWT system avoids the problems mentioned above. Foryou NPWT system records real-time operating data of key components and alarms for potential risks which helps to troubleshoot in time. Both our patients and customers would benefit from the reliability of Foryou NPWT system.
Leakage Rate Monitoring
A reliable seal is indispensable for an effective therapy. Because of the irregular wound shape or wound position, it is difficult for health care givers to maintain a good seal at the wound site.
The leakage rate monitoring of Foryou NPWT system assists health care givers in checking and resolving leakage problem and maintain a reliable seal.
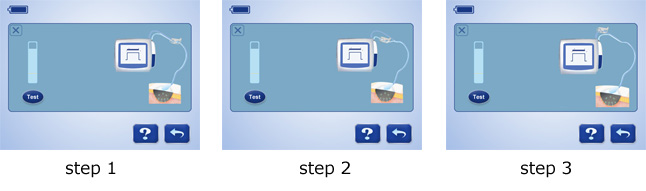
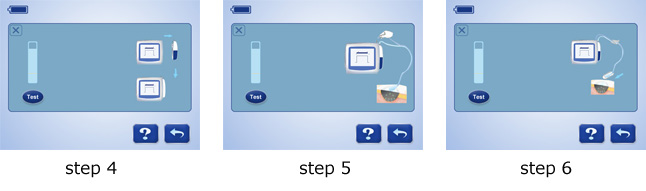
7 steps to troubleshoot leakage:
R&D Team
Patents
Clinical Cases
Copyright © 4L Health Co., Ltd. & Foryou Medical Electronics Co., Ltd. All Rights Reserved.

